The COVID-19 pandemic exposed vulnerabilities in the pharmaceutical supply chain. The US, for example, is dependent on imports for key pharmaceutical products and active pharmaceutical ingredients (APIs). The White House highlights that 87% of the generic API facilities the US depends on are located abroad. Studies show Chinese suppliers of APIs could not work at full capacity during the pandemic, and India reportedly stopped exporting multiple active drug ingredients, fearing domestic shortages. To mitigate risks, the biotechnology and pharmaceuticals industry has to look at diversified sourcing strategies and stringent supply chain audits.

Biopharma’s growing emphasis on building a resilient supply
Regulatory scrutiny and COVID-related concerns drive biotechnology and pharmaceutical entities to invest in strong supply chains
Value chain: upstream
Biotechnology & pharmaceuticals
AT A GLANCE
The COVID-19 pandemic exposed vulnerabilities of the pharma supply chain, highlighting a need for diversified sourcing and leading to regulatory scrutiny.
US policy trends focus on building more resilient supply chains and investing in domestic production.
Increased transparency through regular audits is crucial for building resilient supply chains in the long run.
Policy environment
National and international policy shifts, (e.g. the U.S. National Biotechnology and Biomanufacturing Initiative) aim to strengthen supply chain resilience with strategic investments. US policy trends focus on domestic pharmaceutical manufacturing capabilities; a 2022 U.S. Public Health report highlights the government's focus on supply chain diversification. In the EU, the industry has shown willingness towards reshoring production of APIs to Europe if high costs are addressed.
Supply chain risks
Eli Lilly and AbbVie have challenges in single-source dependencies that can lead to operational disruptions. Non-compliance with quality and rigorous audit trails can mean erosion of stakeholder and consumer trust. Companies have taken steps to mitigate supply chain risks. AbbVie conducts critical supplier quality assessments and audits at least once in three years, with more frequent audits for higher risk suppliers.

Global perspectives
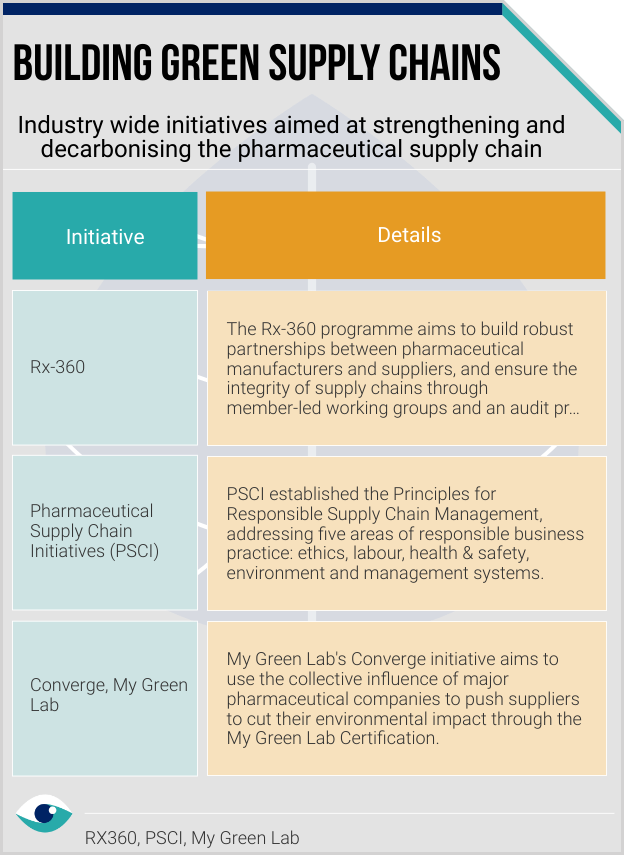
To mitigate disruption and negative impact on their health systems, many countries are adopting the plus-one diversification strategy. A EU’s Policy Department document highlights potential policy actions such as incentivising reshoring and nearshoring, supplier diversification, and expanding domestic production to boost resilience of global supply chains. Biopharma companies are engaging collaboratively through Converge (helping suppliers reduce environmental impact) and the Pharmaceutical Supply Chain Initiative (PSCI), focussed on responsible value chains.
Long-term strategic outlook
Enhancing transparency through periodic audits will be crucial in building resilience and keeping stakeholders informed about how companies are preserving value. Disclosures like the SASB’s HC-BP-430a.1. share information on programmes like the Rx-360 audit programme. Major firms proactively form coalitions to strengthen the supply chain in line with ESG principles. US pharma firm Bristol Myers Squibb highlights the co-founded Manufacture 2030 activate program to help support decarbonisation of API suppliers.
FURTHER READING
- Measures to facilitate the production of APIs (European Parliament)
- Managing critical vendors (Rx360)
- US biotechnology and biomanufacturing initiative (US government)
